How Does Electronegativity Determine Polarity
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Water polar molecule electronegativity partial molecules positive example chemistry hydrogen oxygen molecular charge negative charges bond why covalent biology explain How can i determine bond polarity? + example
Printable Periodic Table Of The Elements Electronegativity | Porn Sex
Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagram The periodic table and periodic trends Electronegativity polarity relative ib chemistry values bonds
Electronegativity and bond polarity
Periodic table of elements electronegativity chart periodic tableElectronegativity pauling periodic table trends values chemistry bonds scale linus electronic number printable atomic period noble gases chemical chart value Electronegativity polarityElectronegativity and polar covalent bonding.
Using electronegativity to determine bond polarityElectronegativity and polarity How does a polar bond differ from a covalent bondChapter 9 section d other aspects of covalent bonds.
Electronegativity polarity
Covalent atoms chemistry differ electron lengths participating asked dependPolar vs. nonpolar bonds — overview & examples Electronegativity (en) & bond polarityPeriodic table.
Electronegativity and polarity study guidePrintable periodic table of the elements electronegativity 7.3 molecular polarity and dipole moments – chemistry fundamentalsCo2 electronegativity carbon molecule polar polarity dioxide non why bond dipole difference oxygen between vector structure bonds molecules charge vectors.

Electronegativity and bond polarity
Electronegativity polarity chart4.2b-electronegativity and polarity Electronegativity rangeElectronegativity polarity chart.
Electronegativity mcgraw polarity covalent moleculesPolarity electronegativity Electronegativity periodic table bond chemistry general polarity ionization elements energy values chemical principles chart pauling bonding applications patterns scale graphCovalent bonding – introductory chemistry.

Electronegativity chart chemistry bonds periodic table covalent elements printable polarity determine atomic section other introductory bond element electronegativities reference sheet
8.7: bond polarity and electronegativityPolar vs. non-polar bonds & molecules Polar covalent bondsPolar vs nonpolar bonds: what is the main difference?.
Nonpolar electronegativity polarity covalent electronPolarity bond dipole electronegativity moment chemistry practice problems Nonpolar bonds electronegativity hydrogen biology oxygen nitrogen electronegative fluorine chemistry overview sylvia freemanBond polarity electronegativity molecular shape covalent ionic bonding chemistry atoms types between different figure two polar nonpolar electron electrons distribution.

Electronegativity polarity chart
Polarity electronegativity bond chemistrySolved use electronegativity to determine bond polarity. 4.2 relative polarity of bonds from electronegativity values [sl ibWhat is electronegativity? + example.
Polarity bond electronegativity atomsElectronegativity and polarity Electronegativity and polarityElectronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common.

Bond polarity, electronegativity and dipole moment
2.2.2 (i,j) electronegativity and bond polarity .
.


Electronegativity and Polarity - cheMYSTERY
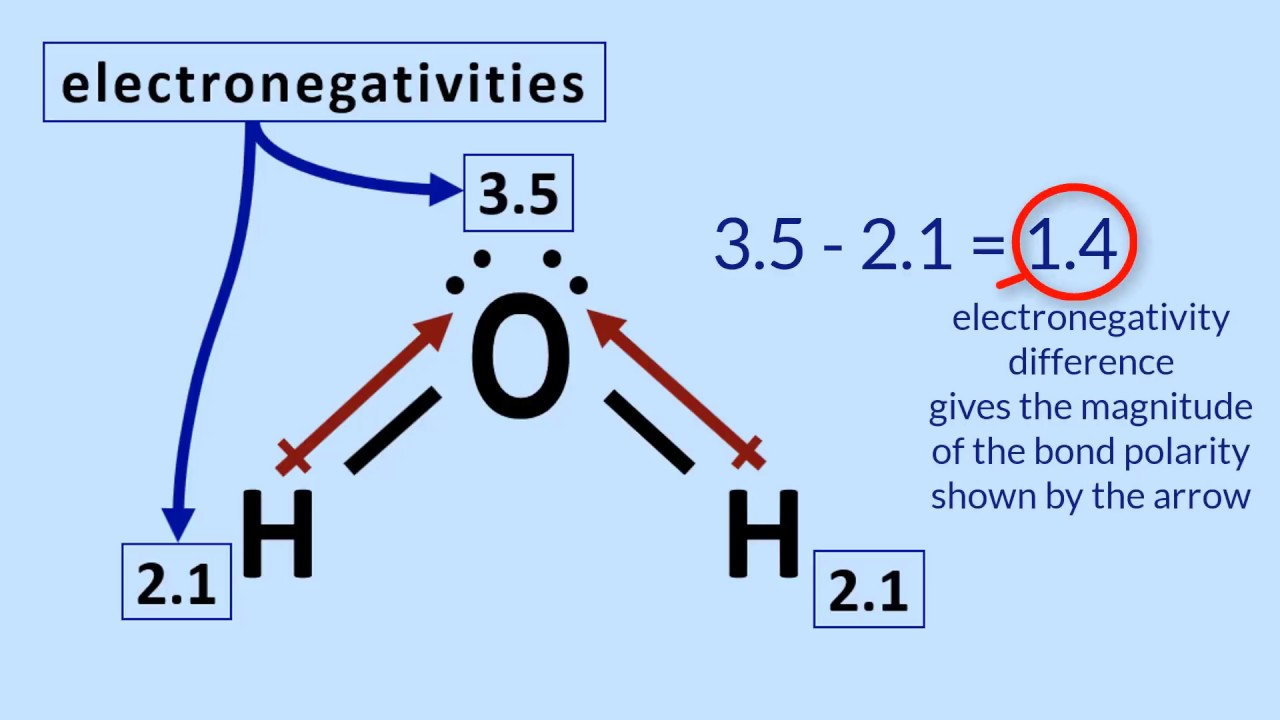
Electronegativity And Polarity Study Guide - gigagoodsite

Solved Use electronegativity to determine bond polarity. | Chegg.com

Polar vs. Non-Polar Bonds & Molecules | ChemTalk

Electronegativity and Polar Covalent Bonding - dummies
Polar vs Nonpolar bonds: What is the Main Difference? - PSIBERG